CE marking means “Conformité Européenne” and consists of a logo with the two letters “C” and “E”. A manufacturer stamps a CE logo on its products to confirm that they comply with the applicable EU safety regulations (protection of human life and of the environment). CE marks may be double-checked by a third party or self-declared by the manufacturer.
CE MARKING FOR STEEL PRODUCTS
WHAT IS “CE MARKING”?
The CE marking, standing for “Conformité Européenne” (European Conformity), is a mandatory conformity mark for certain products within the European Economic Area (EEA). It signifies that the product meets high safety, health, and environmental protection requirements, and, in the case of steel products, it is a declaration from the manufacturer that these products comply with the relevant European product standards and performance characteristics.

The term initially used was “EC Mark” but this terminology was officially replaced by the term “CE Marking” after the issuance of Directive 93/68/EEC back in 1993.
RELEVANCE OF “CE MARKING”
The construction industry, which extensively uses steel products, is a sector where safety and reliability are paramount. Steel components like beams, columns, reinforcements, and other structural elements are fundamental in ensuring the stability and longevity of buildings and infrastructure. The CE marking on these steel products indicates that they adhere to the European Union’s standards for safety, durability, and quality, which are some of the strictest in the world.
Besides steel for structural applications, other steel products (including steel pipes) must be “CE marked” to be legally distributed and installed within the countries of the European Union. This is particularly crucial for imported steel products, for example of Chinese and Indian origin, as non-CE marked products may be considered illegal and subject to sanctions, for both the manufacturer/distributor and the end-user.
BENEFITS OF CE MARKING FOR STEEL PRODUCTS
The CE marking on steel products brings several benefits:
Access to the European Market: Products bearing the CE mark can be freely traded within the EEA, removing barriers to entry in different countries within this zone.
Assurance of Quality: The CE mark provides stakeholders, including engineers, architects, and constructors, with the assurance that the steel products they use comply with the highest European standards for safety and quality.
Competitive Advantage: For manufacturers, obtaining the CE mark can offer a competitive advantage in the market, as it signals to customers that their products are of high quality and meet stringent EU regulations.
Risk Reduction: Using CE-marked steel products reduces the risk of structural failures and the associated liabilities, as these products have been rigorously tested and assessed for safety and performance.
HOW TO ACHIEVE CE MARKING
To achieve CE marking, steel products must undergo a series of evaluations and tests to confirm that they meet the specific technical standards set out by the European Union. This process involves:
Assessment of Production Control Systems: Manufacturers must implement a Factory Production Control (FPC) system. This system ensures that the manufacturing process is consistent and that every product meets the specified quality standards. The FPC system must be assessed by a notified body, an organization designated by an EU country to assess the conformity of products before being marketed.
Testing of Products: Steel products might be subjected to various tests, including mechanical properties, chemical composition, and dimensional accuracy, depending on the specific requirements of the harmonized European standards they need to comply with.
Technical Documentation: Manufacturers must compile technical documentation that provides evidence of conformity. This documentation includes test results, product descriptions, technical drawings, and calculations.
Declaration of Performance (DoP): Once the product has been assessed, the manufacturer must draft a Declaration of Performance (DoP), which details the product’s essential characteristics and its performance as per the EU standards.
Affixing the CE Mark: Following the Declaration of Performance, the manufacturer can then affix the CE mark to their steel products, packaging, or accompanying documents. The CE mark must be visible, legible, and indelible.
The manufacturer has to follow several administrative steps to obtain a CE mark for its products. Depending on the product and the nature of the risks it involves, the manufacturer shall:
- Determine if any directives apply to the product. If more than one applies, the manufacturer will have to comply with any of them.
- Determine the extent to which the product complies with the essential requirements for design and manufacturing in the applicable directive(s).
- Choose the conformity assessment procedure from the options called out by the directive for the product. There are several modules available for the Conformity Assessment Procedures as listed below:
Module A: internal production control
Module Aa: the intervention of a Notified Body
Module B: EC type-examination
Module C: conformity to type
Module D: Production Quality Assurance
Module E: Product Quality assurance
Module F: product verification
Module G: unit verification
Module H: full quality assurance
The directives often use a series of questions about the nature of your product to classify the level of risk and refer to a chart called “Conformity Assessment Procedures”.
The chart includes all of the acceptable options available to a manufacturer to certify their product and affix the CE Marking.
SELF OR THIRD-PARTY CE CERTIFICATION?
LOW-RISK PRODUCTS (AUTOCERTIFICATION)
Auto-certification for CE marking is a simplified procedure where the manufacturer declares that their product meets the necessary EU regulatory requirements, without the need for an independent third-party assessment by a notified body. This process is applicable under certain conditions and for specific product categories where the potential risk to health, safety, and environment is considered lower (low-risk products). Understanding when auto-certification is allowed can help manufacturers navigate the complexities of EU regulations efficiently.
Criteria for Auto-certification
Product Category and Risk Profile: Auto-certification is primarily allowed for products that fall under directives or regulations with a lower perceived risk. The EU legislation identifies these categories, outlining the conditions under which a manufacturer can self-declare their product’s conformity.
Harmonized Standards: The product must comply with the relevant harmonized European standards. These standards provide technical specifications and guidelines ensuring that products meet essential health, safety, and environmental protection requirements. Compliance with these standards simplifies the demonstration of conformity.
Conformity Assessment Procedures: The specific EU directive or regulation governing the product determines the applicable conformity assessment procedures. For low-risk products, these procedures might not require the involvement of a notified body, allowing manufacturers to conduct the necessary assessments and tests in-house.
Steps for Auto-certification
Internal Production Control: The manufacturer needs to have a thorough internal production control system in place. This system should ensure that all manufactured products consistently meet the required EU standards and regulations.
Technical Documentation: Manufacturers must compile and maintain comprehensive technical documentation. This documentation should provide detailed information on the design, manufacture, and operation of the product, demonstrating how the product meets the relevant requirements.
EU Declaration of Conformity (DoC): The manufacturer drafts an EU Declaration of Conformity (DoC), a legal document in which the manufacturer formally declares that their product complies with all applicable EU requirements. The DoC must include information such as the manufacturer’s details, product description, and the specific directives and standards the product complies with.
Affixing the CE Mark: Following the completion of the DoC, the manufacturer can affix the CE mark to their product, packaging, or accompanying documentation. This mark must be visible, legible, and permanent.
Products Typically Eligible for Auto-certification
Simple Pressure Vessels: These are subject to specific conditions under the Simple Pressure Vessels Directive.
Personal Protective Equipment (PPE): Certain categories of PPE that are considered to have a lower risk can also undergo self-certification, depending on the specifics of the PPE Regulation.
- Non-critical products: it is reasonable to include in products eligible for auto certification any device/tool/assembly that could not threaten anyone’s health and safety (most structural pipes, sheets, plates, and other steel would be considered “critical” and therefore subject to third-party assessment instead)
Benefits and Responsibilities
Auto-certification streamlines the process of placing products on the EU market, reducing time and costs associated with third-party assessments. However, it places the full responsibility for compliance on the manufacturer. Any non-compliance identified can lead to penalties, including product recalls and bans from the EU market. Therefore, manufacturers must ensure rigorous adherence to all relevant standards and regulations when opting for auto-certification.
In summary, auto-certification for CE marking is a valuable process for manufacturers of low-risk products, allowing them to self-declare compliance with EU regulations. This approach demands a high level of diligence from manufacturers in meeting the required standards and maintaining proper documentation, ensuring that their products are safe, reliable, and compliant.
HIGH-RISK PRODUCTS
For products considered high-risk, and we would include most steel products in this category, the involvement of a Notified Body is mandatory to obtain CE Marking. A Notified Body is an organization designated by EU countries to assess the conformity of certain products before being placed (sold/installed) on the market. The process involves:
- Identifying Relevant Directives and Standards: As with self-certification, the first step is identifying which directives and standards apply to the product.
- Engaging a Notified Body: The manufacturer must choose an appropriate Notified Body to assess product conformity. The choice of Notified Body can depend on the product type and the services the body is accredited to perform.
- Product Testing and Assessment: The Notified Body conducts or oversees the testing and assessment of the product to ensure it meets the EU requirements. This may involve examining the technical design, and manufacturing process, and performing specific tests.
- Technical Documentation Review: The Notified Body reviews the technical documentation prepared by the manufacturer to ensure it meets compliance requirements.
- Issuance of a CE Certificate: Upon successful assessment, the Notified Body issues a certificate of conformity, allowing the manufacturer to draft the Declaration of Conformity and affix the CE mark to their product.
Products requiring Notified Body Assessment often include those where failure could pose a significant risk to human health and safety.
A “Notified Body” is an organization that has been nominated by a Member Government of the European Union and has been notified by the European Commission. Notified bodies serve as independent test labs and perform the steps called out by the applicable directives. They must have the necessary qualifications to meet the testing requirements outlined in the directives.
Notified bodies may be a private sector organization or a government agency.
Manufacturers may choose a notified body from any member state of the European Union, regardless of the country of the final destination of the goods within the EU. The lists of notified bodies responsible for different products are published by the European Commission in the Official Journal of the European Communities.
A Notified Body is usually able to offer some of the services required to obtain CE Marking:
- product testing
- type-examination certificate issue
- Technical File and design dossier evaluation
- surveillance of product and quality system
- identification of standards
If the products need to be certified by a Notified Body, then the following steps have to be followed:
- Select the applicable product standards and test methods for your product and select a Notified Body.
- Establish an Authorized Representative in the European Union for your product: Some directives require that a manufacturer designates in the European Union an authorized representative to produce Technical Documentation (sometimes called Technical File) in a timely fashion when called upon to do so. The CE Marking itself is not meant to provide details about the product to Surveillance Authorities.
- Technical Documentation (Technical File): The directives require for many products that a Technical Documentation (Technical File) be prepared by the manufacturer. The Technical Documentation (Technical File) holds information that verifies that the testing was conducted properly and that the product complies with applicable standards.
- Prepare a Declaration of Conformity.
The Declaration of Conformity must contain information adequate for tracing the product back to the manufacturer or the authorized representative in the European Union. It may include a list of the directives and standards that your product conforms to, product identification, the manufacturer’s name, address, and signature. - Register your product in the EU
Many products, for instance, Class I Medical Devices, are required to be registered in the EU and, if proven, get a Certificate of Registration. Without this Certificate of Registration, the products are NOT allowed to be affixed with the CE Marking and be placed on the market. - Affix the CE Marking to your product.
There are specific rules to adhere to for the CE Marking. These rules address the size and location of the Marking; affixing the CE Marking to products, packaging, and material or documents shipped with the product; and specific limitations on when and who is permitted to affix the CE Marking.
LOW OR HIGH-RISK PRODUCT?
If there are uncertainties regarding the classification of products and the requirements for certification within the EU, manufacturers or distributors must seek advice from accredited professionals before exporting steel products to the EU. Ensuring regulatory compliance with the CE Marking directives benefits both the seller and their EU clientele by avoiding penalties associated with non-compliance. Lack of knowledge does not serve as a valid defense, and diligence is essential. This is especially pertinent for suppliers outside the EU, who might be unfamiliar with or only have a limited understanding of these regulations.
SANCTIONS FOR MISSING CE MARKING
When a steel product is found on the European market without the required CE marking, it signifies non-compliance with the European Union’s regulations. The consequences of such non-compliance can be significant for the manufacturer, importer, or distributor involved. These sanctions are designed to maintain the integrity of the market, ensuring that all products meet the EU’s high standards for safety, health, and environmental protection.
The specific nature and severity of sanctions can vary depending on the member state’s legislation and the particular circumstances of the non-compliance, but generally, they include several key measures.
1. Market Withdrawal or Recall
The immediate consequence for non-compliance is typically the withdrawal of the non-compliant product from the European market. If the product is already being used in construction or other applications, a recall may be issued. This action prevents further distribution and use of potentially unsafe or non-conforming products.
2. Fines and Penalties
Manufacturers, importers, or distributors found to be in violation of CE marking regulations may be subject to fines and penalties. These financial sanctions can be substantial, varying from country to country within the EU, and are designed to serve as a deterrent against non-compliance. The amount can depend on the severity of the infraction, the perceived risk to public safety or health, and whether the non-compliance was deemed intentional or negligent.
3. Legal Action
In cases where non-compliance results in serious harm or poses significant risks to health and safety, legal action may be taken against the responsible parties. This could result in criminal charges, especially if there is evidence of willful deceit or negligence leading to injury or environmental damage.
4. Damage to Reputation
Beyond the immediate legal and financial repercussions, companies found non-compliant with CE marking regulations may suffer long-term damage to their reputation. In industries where trust and reliability are paramount, such as construction and engineering, the impact of being associated with non-compliant products can lead to a loss of business and difficulty in establishing future partnerships.
5. Cease and Desist Orders
Authorities may issue cease and desist orders to companies violating CE marking regulations, effectively halting the production, import, or distribution of non-compliant steel products. This can lead to significant disruptions in operations and supply chains.
6. Mandatory Compliance Measures
In some cases, authorities may require companies to take specific actions to come into compliance. This might include undergoing additional testing, certification processes, or restructuring manufacturing practices to meet EU standards.
Ensuring Compliance
Given the significant sanctions associated with non-compliance, it is crucial for manufacturers, importers, and distributors of steel products intended for the European market to thoroughly understand and adhere to CE marking requirements. This includes conducting proper assessment procedures, engaging with notified bodies as necessary, and maintaining comprehensive technical documentation to demonstrate compliance.
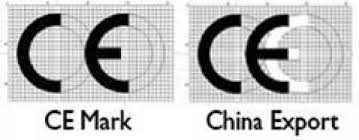
“CE” VS. “CHINA EXPORT” MARKS
The “CE Mark” and the “China Export” mark are two symbols that can appear remarkably similar at first glance but serve entirely different purposes and originate from different regulatory environments. Understanding the distinction between these two marks is crucial for manufacturers, exporters, importers, and consumers to ensure compliance with applicable regulations and to avoid potential confusion or misconceptions regarding product quality, safety, and intended markets.
CE Mark
As discussed above, the CE Mark, standing for “Conformité Européenne” (European Conformity), signifies that a product meets the European Union (EU) standards for health, safety, and environmental protection. The CE Mark is a mandatory conformity marking for products sold within the European Economic Area (EEA), Switzerland, and Turkey. This mark indicates that the product complies with EU legislation and facilitates the free movement of products within the market.
Products requiring the CE Mark cover a wide range, including but not limited to, electrical equipment, machinery, medical devices, toys, and construction products. The process to obtain CE certification involves assessing the product against the relevant EU directives and regulations, which may include safety tests, quality checks, and conformity assessments by notified bodies for certain categories of products.
China Export Mark
The China Export mark, which is often mistakenly referred to as a symbol similar to the CE Mark, is not an officially recognized certification mark. This misconception arises because the “China Export” mark is perceived to use a logo that closely resembles the CE Mark, with the letters “C” and “E” appearing to stand for China Export. However, there is no formal mark that designates “China Export” in the way the CE Mark denotes European conformity. The confusion typically stems from the similarity in the design of certain marks on products manufactured in China, which can unintentionally mimic the CE logo’s appearance.
Key Differences
- Purpose: The CE Mark is a regulatory mark signifying European conformity, whereas the so-called China Export mark does not have a recognized or standardized meaning related to product conformity or regulatory compliance.
- Regulatory Authority: The CE Mark is overseen by the European Union, with strict regulations and standards that must be met for products to be sold within the EEA. The China Export mark, as it is commonly misunderstood, lacks a governing body or formal regulations.
- Geographical Area: The CE Mark is relevant for products intended for the European market, ensuring they comply with EU standards. The notion of a China Export mark does not pertain to a specific regulatory requirement or geographical area.
Conclusion
It is essential for stakeholders in the manufacturing, distribution, and sale of products to recognize the difference between the CE Mark and the misunderstood concept of a China Export mark. Ensuring that products bear the correct CE Mark, when required, is crucial for compliance with European regulations. Manufacturers and exporters must be vigilant to avoid mislabeling or confusion that could lead to regulatory issues or diminish consumer trust. Understanding these distinctions helps prevent potential non-compliance with European safety and quality standards.


5 Responses
Good information, CE certification permits organization to lawfully advertise and disseminate their item with Market and Mandates, Guidelines. CE certification in Philippines.
You are absolutely correct and thanks for giving information about project procurement . I loved your blog and thanks for publishing this!! I am really happy to come across this exceptionally well written content. Thanks for sharing and look for more in future!! You must also check out Dukeswiremesh.com it has some great insights too.
Electrical Conduit is a tube used to protect and route electrical wiring in a building or structure. Electrical Conduit may be made of metal. And plastic, fiber, or fired clay. Most conduit is rigid although but flexible conduit is used for some purposes. Newtech-pipes providing the Electric Conduit Pipes & Fittings in Pakistan. Newtech-Pipes manufactures a complete range of electrical conduit pipes and fittings. Keeping in view of quality.
Call Us Now: +92333-5665265 / +92 51 4438601-4, Visit Our Website: newtech-pipes.com, Owner Name: Talha kazmi, Email: sales@newtech-pipes.com
Electrical Conduit is a tube used to protect and route electrical wiring in a building or structure. Electrical Conduit may be made of metal. And plastic, fiber, or fired clay. Most conduit is rigid although but flexible conduit is used for some purposes. Newtech-pipes providing the Electric Conduit Pipes & Fittings in Pakistan. Newtech-Pipes manufactures a complete range of electrical conduit pipes and fittings. Keeping in view of quality.
Call Us Now: +92333-5665265 / +92 51 4438601-4, Visit Our Website: newtech-pipes.com, Owner Name: Talha kazmi, Email: sales@newtech-pipes.com
WE ARE MANUFACTURE ERW M S STEEL PIPES AND HOLLOW SECTION.WE WANT BUSINESS WITH EUROPE REGION SO REQUIRED CE MARK.SO HELP US WHICH DIRECTIVE OF OUR PRODUCT? PROCESSOR SHARE STEP BY STEP